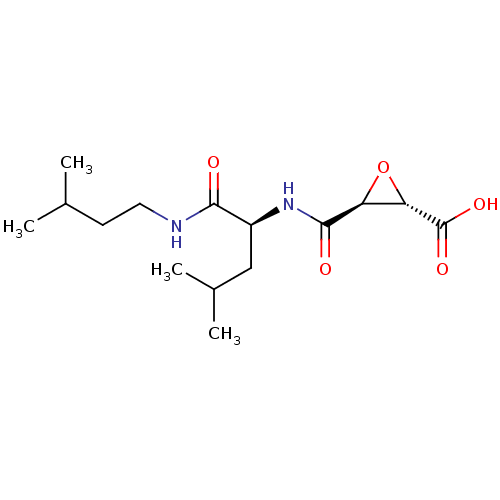
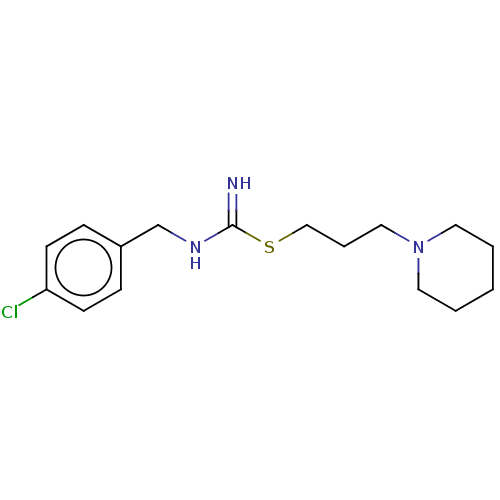
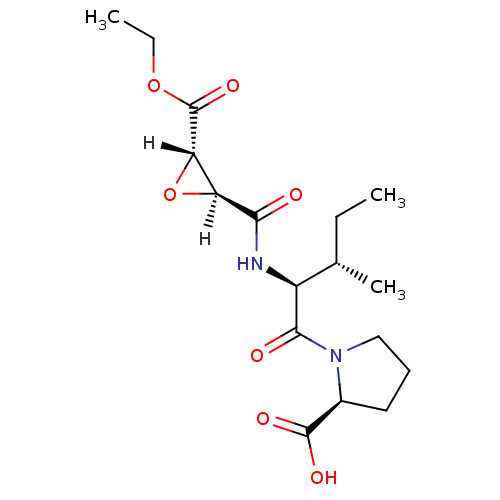
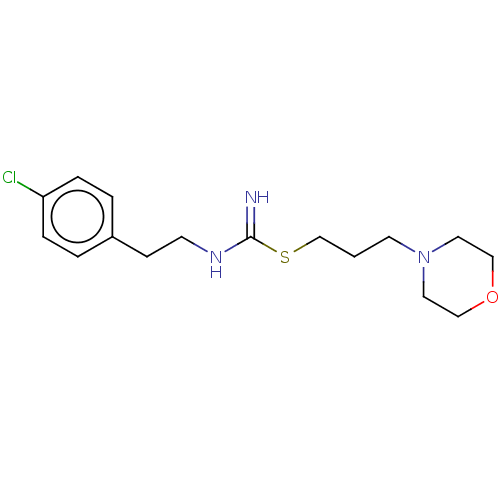
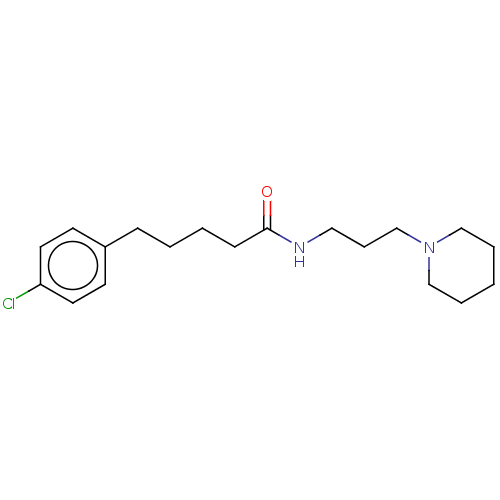
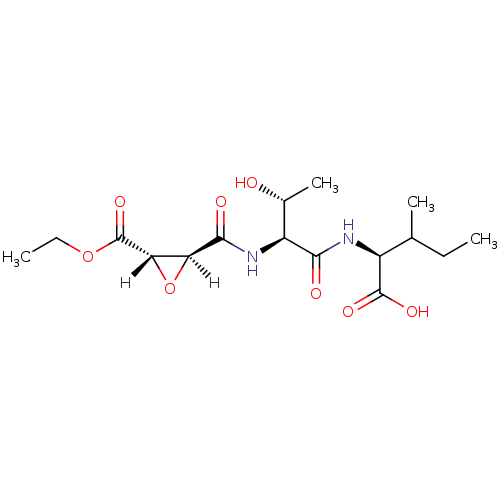
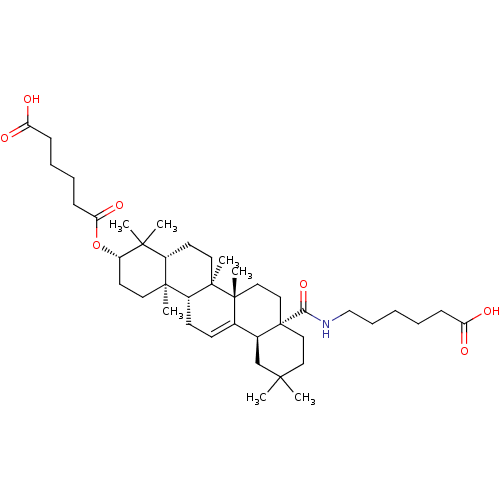
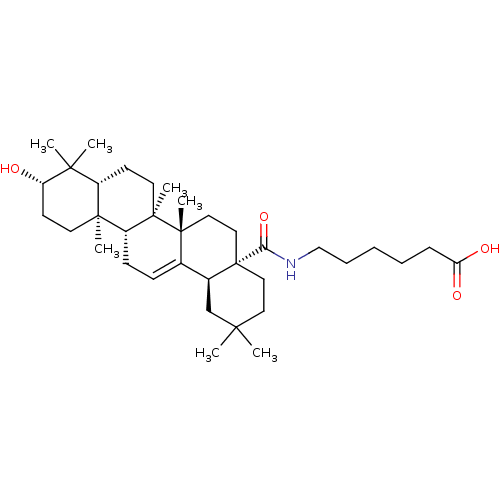
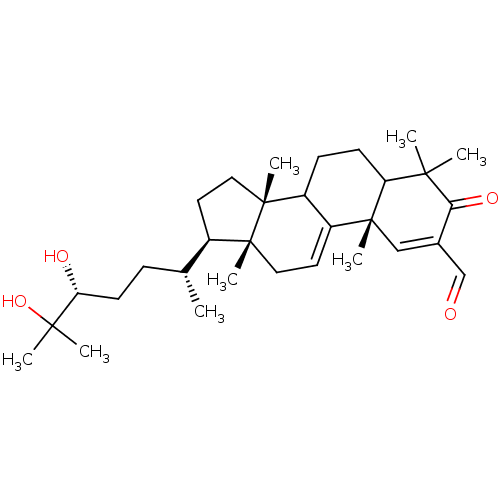
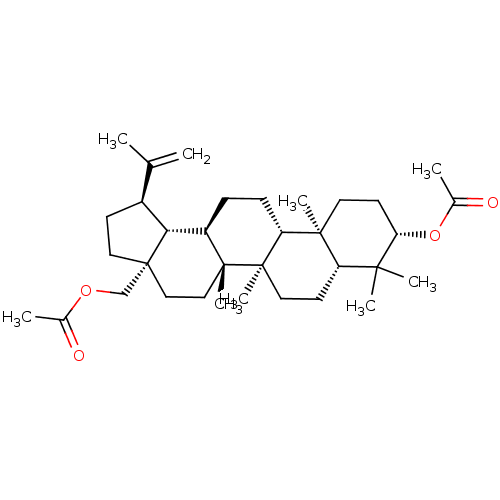
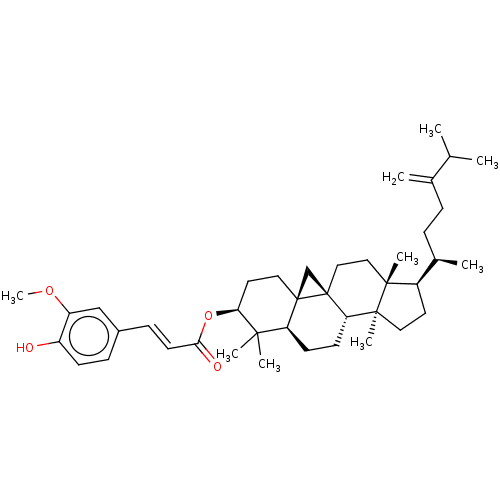
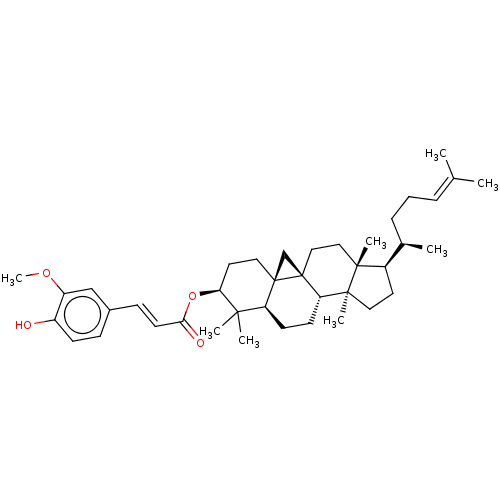
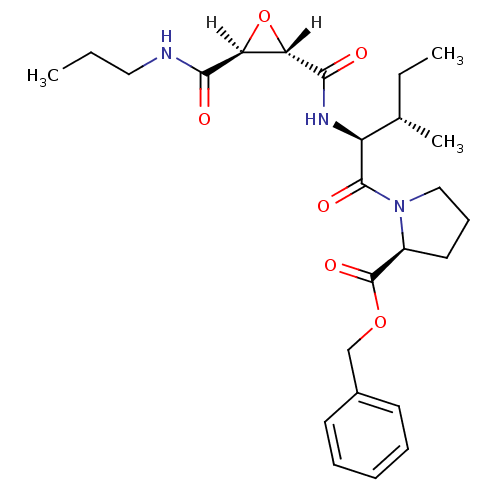
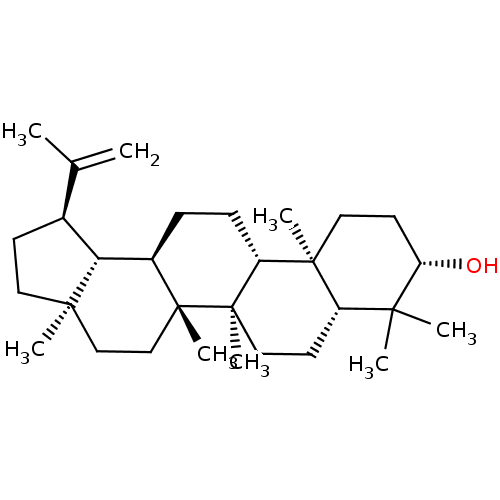
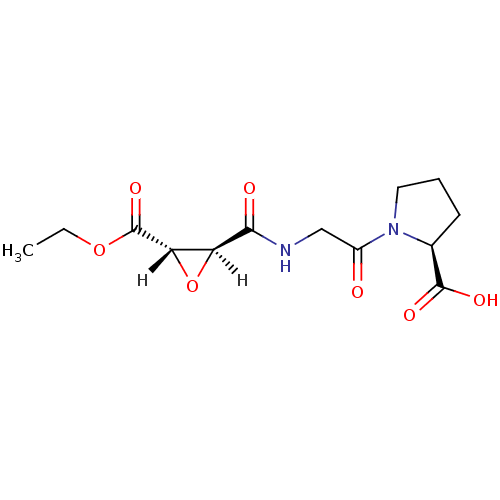
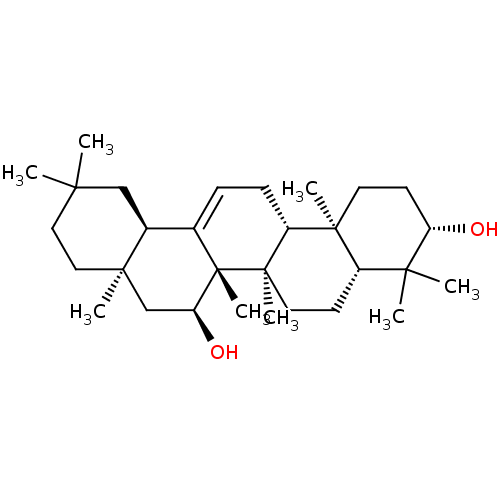
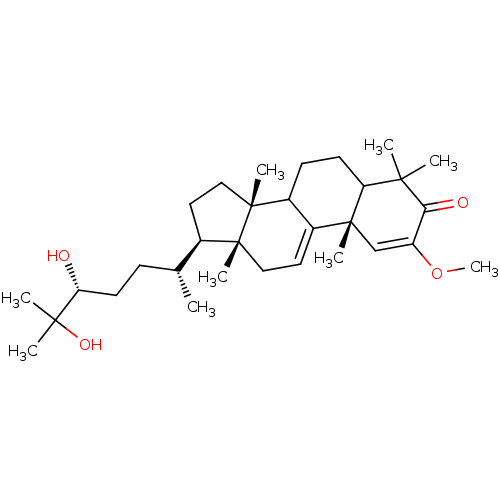
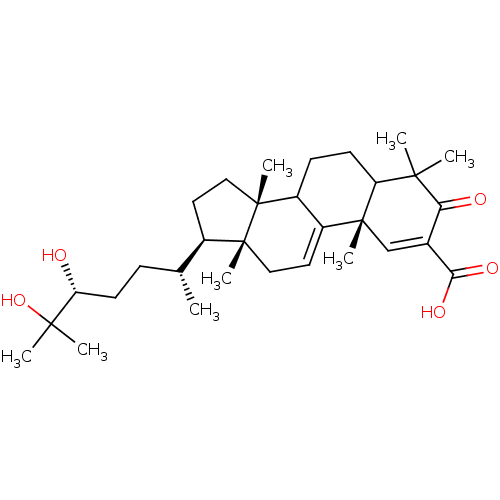
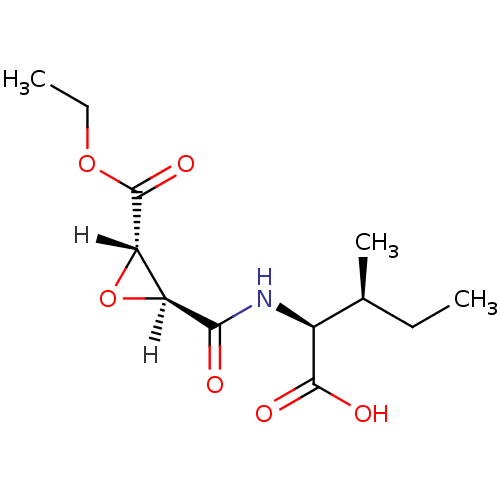
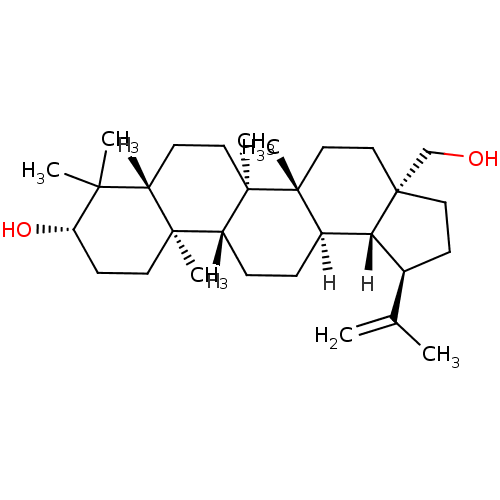
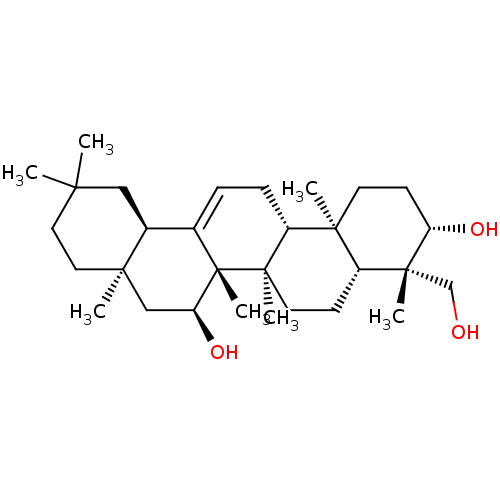
TargetHistamine H3 receptor(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
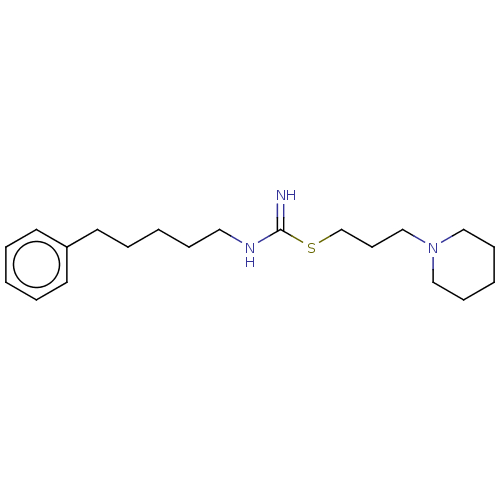
Affinity DataIC50: 0.794nMAssay Description:Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac...More data for this Ligand-Target Pair
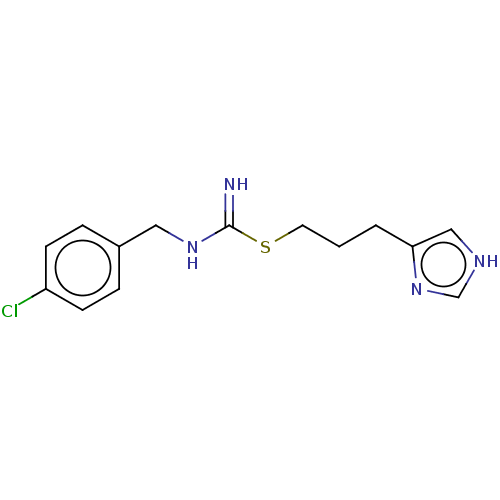
TargetHistamine H3 receptor(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
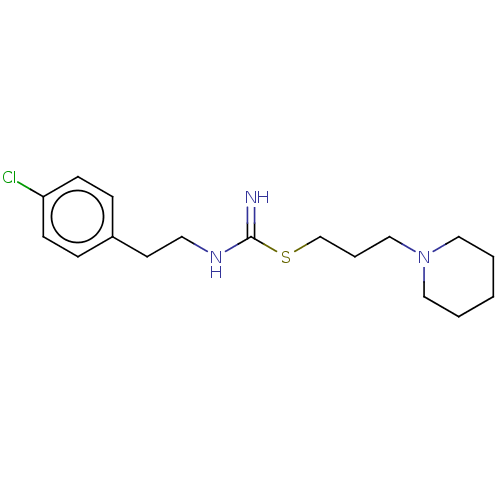
Affinity DataIC50: 2nMAssay Description:Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac...More data for this Ligand-Target Pair
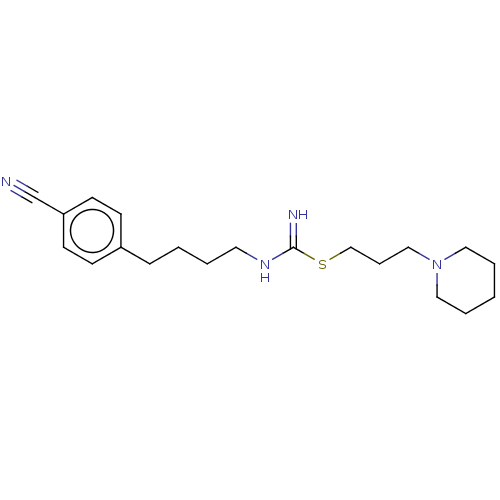
TargetHistamine H3 receptor(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 6.30nMAssay Description:Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac...More data for this Ligand-Target Pair
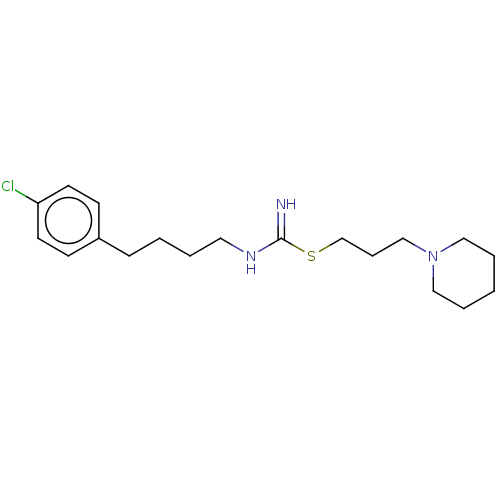
TargetHistamine H3 receptor(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 6.30nMAssay Description:Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 6.30nMAssay Description:Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 7.90nMAssay Description:Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac...More data for this Ligand-Target Pair
Affinity DataIC50: 23nMpH: 6.0 T: 2°CAssay Description:Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 24nMpH: 6.0 T: 2°CAssay Description:Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 29nMpH: 6.0 T: 2°CAssay Description:Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac...More data for this Ligand-Target Pair
Affinity DataIC50: 38nMpH: 6.0 T: 2°CAssay Description:Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMpH: 6.0 T: 2°CAssay Description:Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 79nMAssay Description:Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac...More data for this Ligand-Target Pair
Affinity DataIC50: 120nMpH: 6.0 T: 2°CAssay Description:Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 126nMAssay Description:Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 126nMAssay Description:Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 316nMAssay Description:Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 316nMAssay Description:Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac...More data for this Ligand-Target Pair
Affinity DataIC50: 410nMpH: 6.0 T: 2°CAssay Description:Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 1.26E+3nMAssay Description:Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 1.59E+3nMAssay Description:Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac...More data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
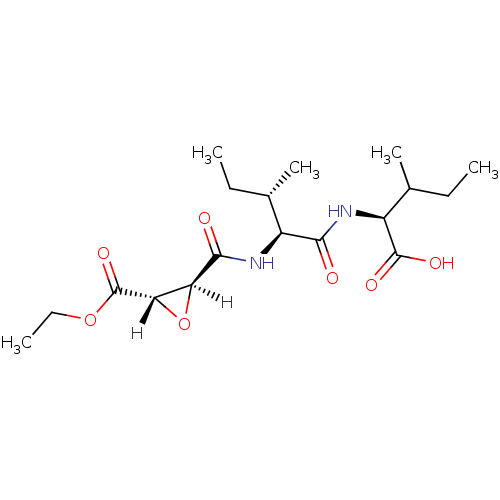
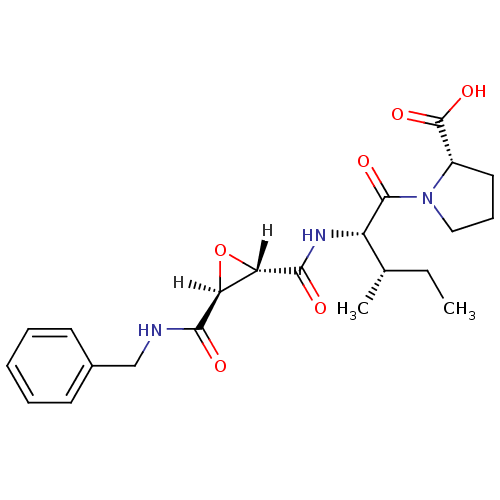
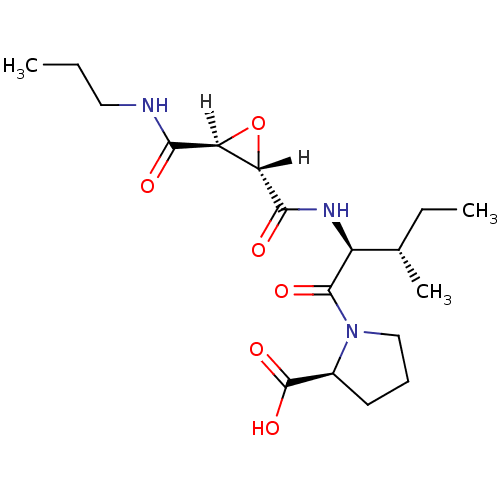
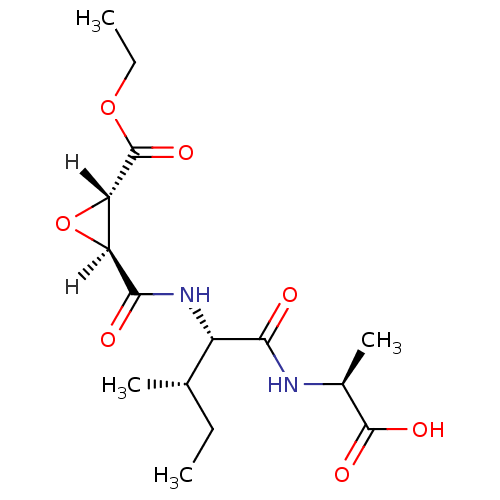
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of HIV1 protease activityMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of HIV1 protease activityMore data for this Ligand-Target Pair
TargetDNA topoisomerase 2-alpha/2-beta(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 1.86E+3nMAssay Description:Inhibitory concentration against human DNA topoisomerase IIMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 2.65E+3nMAssay Description:Antiviral activity against HIV1 Reverse transcriptase activityMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 3.07E+3nMAssay Description:Antiviral activity against HIV1 Reverse transcriptase activityMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 3.64E+3nMAssay Description:Antiviral activity against HIV1 Reverse transcriptase activityMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of HIV1 protease activityMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of HIV1 protease activityMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of HIV1 protease activityMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 4.03E+3nMAssay Description:Antiviral activity against HIV1 Reverse transcriptase activityMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 4.94E+3nMAssay Description:Antiviral activity against HIV1 Reverse transcriptase activityMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of HIV1 protease activityMore data for this Ligand-Target Pair
TargetHistamine H3 receptor(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 6.31E+3nMAssay Description:Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac...More data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of HIV1 protease activityMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of HIV1 protease activityMore data for this Ligand-Target Pair
Affinity DataIC50: 9.10E+3nMpH: 6.0 T: 2°CAssay Description:Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor...More data for this Ligand-Target Pair
TargetDNA topoisomerase 2-alpha(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 1.04E+4nMAssay Description:Inhibition of human 1 unit topoisomerase 2alpha catalytic activity assessed as relaxation of 198 ng supercoiled pBR322 DNA by agarose gel electrophor...More data for this Ligand-Target Pair
Affinity DataIC50: 1.53E+4nMpH: 6.0 T: 2°CAssay Description:Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor...More data for this Ligand-Target Pair
TargetDNA topoisomerase 2-alpha(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 1.75E+4nMAssay Description:Inhibition of human 1 unit topoisomerase 2alpha catalytic activity assessed as relaxation of 198 ng supercoiled pBR322 DNA by agarose gel electrophor...More data for this Ligand-Target Pair
TargetDNA topoisomerase 2-alpha/2-beta(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 1.83E+4nMAssay Description:Inhibitory concentration against human DNA topoisomerase IIMore data for this Ligand-Target Pair
TargetDNA topoisomerase 2-alpha/2-beta(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 2.27E+4nMAssay Description:Inhibitory concentration against human DNA topoisomerase IIMore data for this Ligand-Target Pair
TargetDNA topoisomerase 2-alpha/2-beta(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 2.38E+4nMAssay Description:Inhibitory concentration against human DNA topoisomerase IIMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+4nMpH: 6.0 T: 2°CAssay Description:Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor...More data for this Ligand-Target Pair
TargetDNA topoisomerase 2-alpha(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 3.86E+4nMAssay Description:Inhibition of human 1 unit topoisomerase 2alpha catalytic activity assessed as relaxation of 198 ng supercoiled pBR322 DNA by agarose gel electrophor...More data for this Ligand-Target Pair
TargetDNA topoisomerase 2-alpha(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 3.87E+4nMAssay Description:Inhibition of human 1 unit topoisomerase 2alpha catalytic activity assessed as relaxation of 198 ng supercoiled pBR322 DNA by agarose gel electrophor...More data for this Ligand-Target Pair
TargetDNA topoisomerase 2-alpha(Homo sapiens (Human))
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 4.26E+4nMAssay Description:Inhibition of human DNA topoisomerase 2alphaMore data for this Ligand-Target Pair





 3D Structure (crystal)
3D Structure (crystal)